Polysorbate 80 (Ⅱ)
( NMPA CDE NO.:F20209990115 )
Name: Polysorbate 80 (Ⅱ)
Other Name:Tween 80; Polyoxyeyhylene 20 sorbitanmonooleate
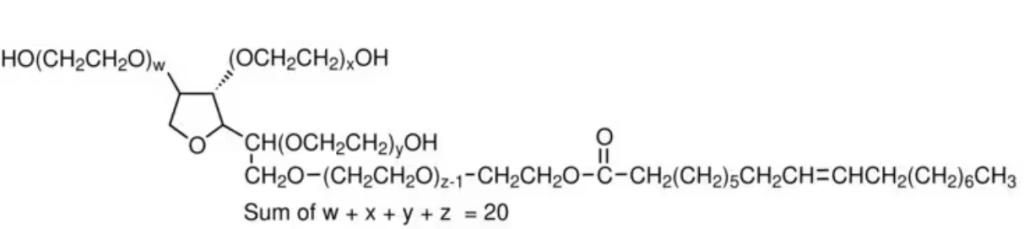
M.F.: C64H124O26
M.W.: 1309.63
CAS No.: 9005-65-6
Packaging:1kg/bottle/carton; 5kg/bottle/carton; 20kg/drum/carton
Quality Standard: USP-NF; EP; ChP;JP;BP.
Appearance: Colorless to light yellow viscous liquid.
Freely soluble in water, ethanol, methanol, ethyl acetate; Very slightly soluble in mineral oils.
Advantages: Compared with Polysorbate 80 (CP):
1.The source of fatty acid is clearly plant-derived Oleic Acid.
2.It has lower acid value, peroxide value, moisture, and residue on ignition.
3.Fatty acid content: Oleic Acid content is not less than 98.0%, much higher than Polysorbate (CP) requirements of 58.0%; The content of other fatty acids did not exceed 0.5%.
4.The amount of endotoxin contained in 1mg Polysorbate 80(Ⅱ) should be less than 0.012EU.
Polysorbate 80 (Ⅱ) is suitable for injection and is superior to ordinary Polysorbate 80 in terms of purity, impurity control, safety, and application scenarios, with higher quality requirements.
Application:
Polysorbate 80 (Ⅱ) is a non-ionic surfactant with an HLB value of 15. It can be used as an solubilizing agent, wetting agent, plasticizer, and O/W emulsifier.
The molecular structure of polysorbate 80 (Ⅱ) contains both hydrophilic and hydrophobic groups. By forming spherical micelles, it reduces the interfacial tension between oil and water and inhibits crystallization, thereby increasing the solubility of the drug;At the same time, it can also make the oil phase evenly dispersed in the water phase to prevent delamination or aggregation, and can also reduce protein denaturation in biological agents to maintain drug activity;Polysorbate 80 (Ⅱ) can also promote the transmembrane transport of drugs, increase the bioavailability of drugs, and sometimes it can encapsulate drug molecules, reducing the irritancy of drugs.
| Application | Function | Example |
| Injection | Solubilizing agent | Docetaxel Injection |
| Eye Drop | Avitears™ Lubrication Eye Drops | |
| Vaccine | Protein stabilizer | Ad26.COV2-S COVID-19 suspension for vaccine injection |
Incompatibility: Incompatible with phenol, tannic acid, tar and tar-like substances.
